Definition of Microparticles
Microparticles are small, solid particles typically ranging from 1 to 1000 microns in diameter. These particles can be composed of a variety of materials such as polymers, lipids, proteins, or ceramics. Microparticles are often used in drug delivery systems, biomedical applications, and other industrial purposes. The core function of microparticles is to encapsulate, deliver, or protect active substances, such as drugs, nutrients, or bioactive compounds, providing controlled release or targeted delivery.
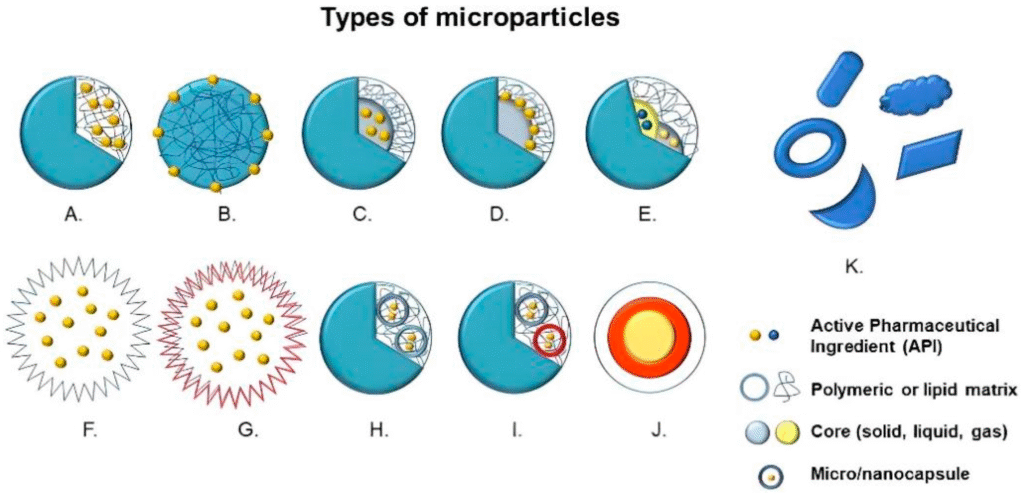
Microparticles can be designed to release their contents gradually over time, or they can provide immediate release, depending on the material and method of preparation. Some common types of microparticles include:
Polymeric Microparticles: Made from biocompatible and biodegradable polymers such as PLGA (poly(lactic-co-glycolic acid)) or PLA (poly(lactic acid)).
Lipid-Based Microparticles: Composed of lipids that form a structure capable of encapsulating hydrophobic or hydrophilic substances.
Protein Microparticles: Used in vaccine delivery or other therapeutic applications.
Microparticles are used in controlled release drug delivery, vaccine delivery, and even in food, cosmetic, and agricultural applications.
Advantages of Microparticles
1. Controlled and Sustained Release: Microparticles allow for controlled release of encapsulated drugs or bioactive agents. This can lead to prolonged therapeutic effects and reduce the need for frequent dosing, improving patient compliance and convenience.
2. Improved Stability: By encapsulating sensitive active ingredients such as drugs, proteins, and enzymes, microparticles protect these substances from degradation due to environmental factors like moisture, heat, or light. This increases the shelf life and stability of the active compound.
3. Targeted Drug Delivery: Microparticles can be engineered to target specific tissues or organs, ensuring that the active substance is delivered directly to the site of action. This improves the therapeutic efficacy and reduces side effects on healthy tissues.
4. Protection of Active Ingredients: Microparticles serve as protective carriers for sensitive substances, shielding them from degradation or inactivation by external factors like gastric acid, enzymes, or oxidative stress.
5. Biocompatibility and Biodegradability: Many microparticles are made from biocompatible and biodegradable materials, such as certain polymers and lipids, which are safely absorbed or eliminated by the body without causing harm. This reduces the risk of toxicity.
6. Versatility in Formulation: Microparticles can be used to encapsulate a wide range of substances, including small molecules, proteins, vaccines, and even cells. This versatility makes them useful in many fields, including pharmaceuticals, nutraceuticals, food products, and cosmetics.
7. Reduced Toxicity and Side Effects: The use of microparticles for drug delivery can reduce systemic side effects by allowing for localized release of the drug at the target site, minimizing exposure to healthy tissues.
8. Improved Bioavailability: Microparticles can enhance the bioavailability of poorly soluble or unstable drugs by improving their solubility and stability, increasing their effectiveness in the body.
9. Combination Therapy: Microparticles can be used to encapsulate multiple drugs or therapeutic agents, allowing for combination therapy. This is beneficial in treating complex diseases, such as cancer, where multiple drugs need to be delivered simultaneously.
Disadvantages of Microparticles
1. Production Complexity: The process of creating microparticles involves sophisticated techniques, such as solvent evaporation, spray drying, or coacervation, which require precise control over factors like particle size, drug loading, and release profile. This can lead to production challenges.
2. High Production Costs: The cost of manufacturing microparticles can be high due to the need for specialized materials, equipment, and techniques. This increases the overall cost of microparticle-based drug delivery systems, making them more expensive than traditional formulations.
3. Encapsulation Efficiency: Achieving high encapsulation efficiency (i.e., effectively incorporating the active ingredient into the microparticle) can be challenging. Low encapsulation efficiency leads to poor drug delivery and loss of material.
4. Drug Release Profile Variability: The release rate of drugs from microparticles may not always be predictable or consistent. Variability in the release profile can result from factors such as the type of polymer used, manufacturing process, and environmental conditions (e.g., pH, temperature), which can complicate their application in controlled release systems.
5. Potential for Toxicity: Some materials used in microparticle formation, such as certain polymers or excipients, may not be fully biocompatible or biodegradable. If these materials accumulate in the body or cause inflammation, they could lead to toxicity or other adverse effects.
6. Size-Dependent Issues: The size of microparticles can affect their distribution and uptake in the body. Microparticles that are too large may not effectively reach the target site or may be cleared quickly by the immune system. Conversely, very small microparticles may not provide adequate control over drug release.
7. Difficulty in Scaling Up: While microparticles may be easy to produce in small laboratory batches, scaling up production for commercial manufacturing can present challenges. Maintaining consistency in particle size, drug loading, and release profiles at a larger scale is often difficult.
8. Limited Shelf Life: Microparticles that encapsulate certain sensitive substances, like proteins or peptides, may have a limited shelf life. Over time, the encapsulated drug may degrade, leading to reduced therapeutic effectiveness.
9. Immune System Interaction: If the microparticles are composed of materials that are not completely biocompatible, they could be recognized by the immune system as foreign bodies, potentially causing an immune response. This could result in inflammation or other complications, especially for long-term use.
Applications of Microparticles
Microparticles are solid particles ranging from 1 to 1000 μm that can encapsulate drugs, proteins, or other bioactive agents. In pharmaceuticals, they are used to improve drug stability, control release, target delivery, and enhance bioavailability. Microparticles can be made from polymers, lipids, or inorganic materials and are a versatile tool in modern drug delivery.
1. Controlled and Sustained Drug Release
Microparticles allow gradual drug release over time, reducing dosing frequency and improving patient compliance.
Examples:
- Theophylline microparticles for sustained asthma therapy.
- Verapamil microparticles for cardiovascular treatment.
Mechanism: Drug is released via diffusion through the polymer matrix or by polymer degradation.
2. Targeted Drug Delivery
Microparticles can deliver drugs to specific tissues, organs, or cells, enhancing efficacy and minimizing systemic side effects.
Examples:
- Doxorubicin microparticles for tumor targeting.
- Microspheres for liver-directed delivery of anticancer drugs.
3. Vaccine Delivery
Microparticles can protect antigens and act as adjuvants to enhance immune response.
Examples:
- Hepatitis B or influenza antigens encapsulated in biodegradable microparticles.
- Experimental microparticle-based cancer vaccines.
4. Injectable Depot Systems
Microparticles allow long-acting injectable formulations, releasing drugs slowly over weeks or months.
Examples:
- Leuprolide microparticles for prostate cancer therapy.
- Risperidone microparticles for long-acting psychiatric treatment.
5. Oral Drug Delivery
Microparticles protect drugs from acidic gastric environments and release them at specific intestinal sites.
Examples:
- 5-Aminosalicylic acid microparticles for colon-specific delivery in ulcerative colitis.
- Insulin microparticles for oral delivery (experimental).
6. Pulmonary Drug Delivery
Microparticles are suitable for inhalable formulations for lung-specific therapy.
Examples:
- Rifampicin microparticles for tuberculosis treatment.
- Albuterol microparticles for asthma.
7. Protein and Peptide Delivery
Microparticles stabilize biopharmaceuticals (proteins, peptides) and improve their bioavailability and half-life.
Examples:
- Insulin microparticles for controlled release.
- Growth hormone microparticles.
8. Taste Masking
Microparticles mask the bitter or unpleasant taste of drugs.
Examples: Pediatric formulations of quinine, ranitidine, or chloroquine.
9. Reduction of Side Effects
By controlling release and targeting specific tissues, microparticles minimize adverse effects.
Examples: NSAID microparticles reduce gastrointestinal irritation.
10. Diagnostic Applications
Microparticles can carry contrast agents or radioactive isotopes for imaging and diagnostics.
Examples:
- Radiolabeled microparticles for liver imaging.
- MRI contrast agent-loaded microparticles.
Common Materials for Microparticles
- Natural polymers: Gelatin, chitosan, alginate, albumin
- Synthetic polymers: PLGA, polycaprolactone, Eudragit
- Lipids: Phospholipids for lipid-based microparticles
- Inorganic materials: Silica, calcium phosphate
Summary Table of Applications
| Application | Examples | Benefits |
| Controlled/sustained release | Theophylline, Verapamil | Reduced dosing frequency |
| Targeted delivery | Doxorubicin, Liver-targeted drugs | Minimizes systemic side effects |
| Vaccine delivery | Hepatitis B antigen, Influenza | Enhanced immune response |
| Injectable depot systems | Leuprolide, Risperidone | Long-acting therapy |
| Oral delivery | 5-ASA, Insulin | Site-specific release |
| Pulmonary delivery | Rifampicin, Albuterol | Targeted lung therapy |
| Protein/peptide delivery | Insulin, Growth hormone | Stabilization & controlled release |
| Taste masking | Quinine, Ranitidine | Improves patient compliance |
| Reduction of side effects | NSAIDs | Minimizes adverse effects |
| Diagnostic applications | Radiolabeled or MRI microparticles | Enhanced imaging |
Conclusion
Microparticles offer significant advantages in controlled release drug delivery, targeted therapy, and protection of sensitive substances. They are versatile and widely used in various applications, including pharmaceuticals, food, and cosmetics. However, the production complexity, cost, and variability in release profiles must be carefully considered when developing microparticle-based systems. Through optimization of the materials, manufacturing processes, and drug formulations, the potential of microparticles can be fully realized, providing effective and reliable drug delivery solutions.

