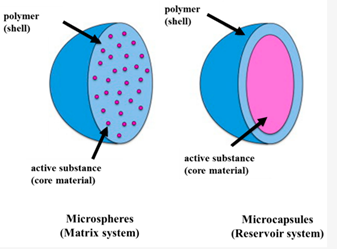
Microencapsulation is a process where active substances (such as drugs, nutrients, or bioactive compounds) are enclosed within a protective coating or shell, typically in the micron or sub-micron size range (1 to 1000 microns). The primary goal is to protect the active ingredient from environmental factors (e.g., light, moisture, air) or to control the release of the active substance over time. The coating material used can be a polymer, lipid, or other biocompatible materials, which are selected based on the desired properties of the final product.
Microencapsulation can be achieved using different techniques, including:
Spray Drying
Coacervation
Pan Coating
Solvent Evaporation
Interfacial Polymerization
Fluidized Bed Coating
These methods result in the formation of microspheres or microcapsules, which can encapsulate a wide range of substances, from pharmaceuticals to food ingredients.
Advantages of Microencapsulation
1. Controlled Release of Active Ingredients: Microencapsulation allows for the controlled release of the encapsulated drug or substance over time. This can provide sustained therapeutic effects, reduce dosing frequency, and minimize side effects by avoiding peak concentrations of the active ingredient.
2. Protection of Active Substances: The encapsulation protects sensitive compounds (e.g., enzymes, proteins, vitamins, or drugs) from degradation due to environmental factors such as heat, light, oxygen, and moisture. This enhances the stability and shelf life of the product.
3. Targeted Drug Delivery: Microencapsulation can be designed to release the encapsulated substance at specific locations within the body, such as in the stomach, intestines, or targeted tissues. This is particularly useful in drug delivery systems, where the aim is to improve therapeutic outcomes while reducing systemic side effects.
4. Improved Bioavailability: By protecting the active ingredient and controlling its release, microencapsulation can improve the bioavailability of poorly soluble or unstable drugs, enabling more effective absorption in the body.
5. Taste Masking: In the pharmaceutical and food industries, microencapsulation can mask the unpleasant taste of certain drugs or ingredients, making them more palatable for patients or consumers.
6. Minimization of Irritation: Microencapsulation can prevent irritation to sensitive tissues by controlling the release of active substances, especially in the case of orally administered drugs that may irritate the stomach lining.
7. Improved Handling and Stability: The encapsulation process can make active ingredients easier to handle, process, and transport by converting them into a solid form that is less prone to degradation.
8. Versatility: Microencapsulation can be applied to a wide range of industries, including pharmaceuticals, nutraceuticals, food, cosmetics, and agriculture, making it a versatile technology.
Disadvantages of Microencapsulation
1. Cost of Production: The microencapsulation process can be expensive due to the specialized equipment required, the cost of encapsulating materials, and the complexity of the manufacturing process. This can result in higher production costs for the final product.
2. Encapsulation Efficiency: The process may not always achieve a high encapsulation efficiency, meaning that some of the active ingredient may not be encapsulated effectively, leading to a loss of product and reduced performance.
3. Release Rate Control Challenges: Achieving precise control over the release rate of the encapsulated substance can be difficult, especially when dealing with highly variable or unstable materials. Variations in temperature, humidity, or the properties of the encapsulating material can affect the release profile.
4. Limited to Specific Types of Substances: Not all substances can be effectively microencapsulated. The process is particularly suitable for substances that are sensitive to environmental conditions or require controlled release, but some materials may not form stable microcapsules or may degrade during the process.
5. Regulatory and Quality Control Issues: Microencapsulated products, especially in pharmaceuticals, face stringent regulatory requirements. The consistency, stability, and performance of the microencapsulated product must be thoroughly tested to meet regulatory standards, which can add complexity to product development and approval.
6. Possible Toxicity of Encapsulating Materials: If the materials used in the encapsulation process are not biocompatible or biodegradable, they could pose risks to health, particularly when used in pharmaceutical applications. The choice of encapsulating material must be carefully considered to ensure safety.
7. Environmental Impact: The production of certain synthetic encapsulating materials may have an environmental impact, especially if the materials are not biodegradable or if the manufacturing process generates waste that is difficult to dispose of safely.
8. Complicated Manufacturing Process:
The microencapsulation process can be technically complex and requires precise control over conditions such as temperature, solvent composition, and processing time to ensure uniformity and quality of the microcapsules.
Applications of Microencapsulation
Microencapsulation is a versatile and powerful technology in pharmaceutical sciences. It involves enclosing active pharmaceutical ingredients (APIs) or other bioactive agents within a protective coating or matrix to form microcapsules, typically ranging from 1 to 1000 µm in size. This technique enhances the stability, bioavailability, and therapeutic efficiency of drugs while allowing controlled release. Here’s a comprehensive look at microencapsulation applications in pharmaceuticals:
1. Controlled and Sustained Drug Release
Microencapsulation allows drugs to be released over extended periods, reducing the frequency of dosing and improving patient compliance.
Examples:
- Theophylline microcapsules for sustained release in asthma treatment.
- Verapamil microcapsules for cardiovascular therapy.
Mechanism: The coating material controls the rate of drug diffusion or erosion, allowing a steady release into the bloodstream.
2. Targeted Drug Delivery
Microcapsules can be designed to release drugs at specific sites, such as the gastrointestinal tract, colon, or tumor tissues.
Examples:
- Colon-targeted delivery using pH-sensitive polymers for drugs like mesalamine.
- Cancer therapy with doxorubicin-loaded microcapsules targeted to tumor sites.
Benefit: Minimizes systemic side effects and maximizes therapeutic effect at the target site.
3. Protection of Sensitive Drugs
Microencapsulation protects drugs from environmental degradation due to light, heat, moisture, or enzymatic activity.
Examples:
- Peptides and proteins (e.g., insulin) encapsulated in biodegradable polymers like PLGA.
- Probiotics or live microorganisms protected from stomach acid for gut delivery.
4. Taste Masking
Many APIs have a bitter or unpleasant taste. Microencapsulation masks the taste without altering drug efficacy.
Examples:
- Pediatric formulations of quinine, chloroquine, or ranitidine.
Mechanism: The drug is enclosed in a polymer coating that dissolves only after swallowing.
5. Enhancement of Bioavailability
Microencapsulation can improve the solubility and absorption of poorly water-soluble drugs.
Examples:
- Curcumin and resveratrol microcapsules for improved oral absorption.
- Lipophilic vitamins (A, D, E, K) stabilized in microcapsules.
6. Reduction of Side Effects
By controlling the release and targeting specific sites, microencapsulation reduces toxicity associated with high systemic concentrations.
Example: NSAIDs like ibuprofen in microcapsules reduce gastrointestinal irritation.
7. Combination Therapy
Multiple drugs with different release profiles can be encapsulated together for synergistic therapy.
Example: Antihypertensive combinations (e.g., nifedipine and atenolol) in a single microcapsule system.
8. Vaccine Delivery
Microencapsulation is used for oral or mucosal vaccines to protect antigens and enhance immunogenicity.
Example: Encapsulation of hepatitis B surface antigen in biodegradable polymers.
9. Formulation of Inhalable or Injectable Drugs
Microencapsulation allows the preparation of aerosols for pulmonary delivery or injectable depots.
Examples:
- Pulmonary delivery of insulin or antibiotics.
- Long-acting injectable formulations like risperidone microspheres for psychiatric disorders.
10. Stability in Liquid Formulations
Drugs that are unstable in aqueous solutions can be stabilized via microencapsulation.
Example: Vitamins or antioxidants in beverages and syrups.
Common Polymers and Coating Materials
- Natural polymers: Gelatin, alginate, chitosan, dextran
- Synthetic polymers: Poly(lactic-co-glycolic acid) (PLGA), Eudragit, ethylcellulose
- Lipids: Phospholipids for liposomal microcapsules
Conclusion
Microencapsulation is a powerful technology that offers many benefits, particularly in the controlled release and protection of sensitive substances. However, its practical implementation requires careful consideration of the material properties, manufacturing costs, and regulatory requirements. Despite these challenges, microencapsulation continues to be a key tool in various industries, improving product efficacy, stability, and patient compliance in pharmaceuticals and other applications.

