Definition
Quality by Design (QbD) is a systematic approach to pharmaceutical development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.
According to the ICH Q8 guideline:
“QbD is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.”
QbD integrates quality into the product at the design stage rather than relying on testing at the end of production to ensure product quality.
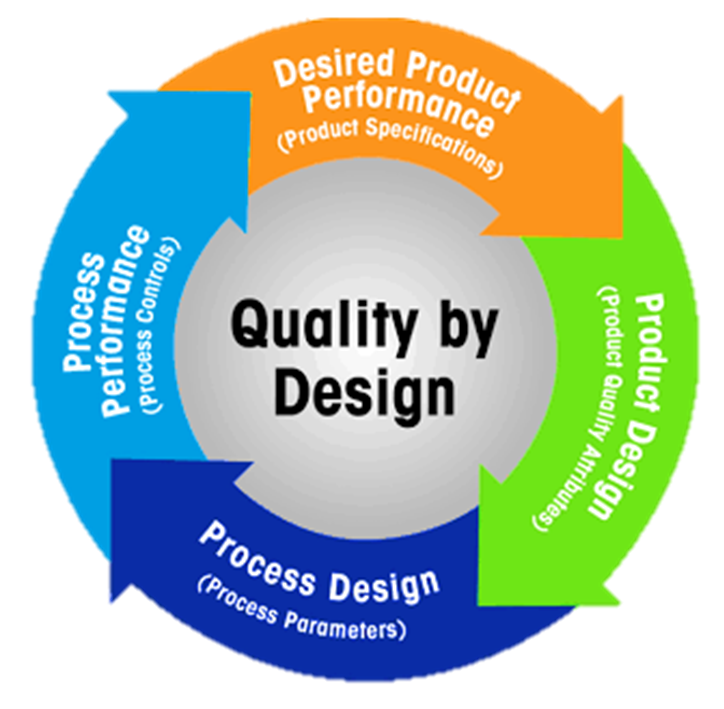
Overview of QbD
Traditionally, quality was ensured through testing and inspection. However, this reactive approach lacked robustness and understanding. QbD shifts the paradigm toward a proactive, science- and risk-based approach that builds quality into products and processes.
Core Principles of QbD
- Product quality should be ensured by design.
- Understanding the process is critical to control variability.
- Risk-based approaches are central to decision-making.
- A well-developed control strategy ensures consistent product quality.
QbD vs Traditional Quality Systems
| Feature | Traditional Approach | Quality by Design |
| Focus | Final product testing | Product and process design |
| Quality assurance | End-point inspection | Built-in during development |
| Process understanding | Limited | Extensive |
| Risk management | Not systematic | Integral |
| Flexibility in regulatory filing | Limited | Greater (Design Space) |
Elements of a QbD Program
The major components or elements of QbD in pharmaceutical development are outlined below:
1. Quality Target Product Profile (QTPP)
A prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy.
QTPP Includes:
- Dosage form
- Route of administration
- Dosage strength
- Pharmacokinetics (PK)
- Stability
- Purity and identity
- Patient compliance and convenience
2. Critical Quality Attributes (CQAs)
Physical, chemical, biological, or microbiological properties that must be controlled within defined limits to ensure product quality.
Examples:
- Content uniformity
- Assay
- Degradation products
- Dissolution
- Moisture content
3. Risk Assessment and Management
Used to identify, evaluate, and prioritize potential risks associated with product and process development.
Common tools:
- Failure Mode and Effects Analysis (FMEA)
- Ishikawa (Fishbone) Diagrams
- Risk Ranking and Filtering
4. Design Space (DS)
A multidimensional combination of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality.
Benefits of DS:
- Regulatory flexibility
- Process robustness
- Fewer post-approval changes
5. Control Strategy
A planned set of controls derived from current product and process understanding that ensures process performance and product quality.
Includes:
- Input material controls
- Process controls
- In-process testing
- Specifications for the final product
6. Process Analytical Technology (PAT)
A system for designing, analyzing, and controlling manufacturing processes through the measurement of critical process parameters that affect critical quality attributes.
Examples:
- Near-infrared spectroscopy (NIR)
- Raman spectroscopy
- On-line HPLC
7. Continual Improvement
Using lifecycle management to collect data and update process understanding post-approval to achieve ongoing product enhancement and innovation.
QbD Tools
To implement QbD successfully, several structured tools and methodologies are employed to ensure a systematic, data-driven, and risk-based approach:
1. Risk Assessment Tools
- FMEA (Failure Mode and Effects Analysis): Identifies potential failure modes and their impact.
- FTA (Fault Tree Analysis): Helps identify root causes of failures.
- HAZOP (Hazard and Operability Study): Used in process design to identify deviations.
2. Design of Experiments (DoE)
A statistical tool used to understand the relationship between input factors (e.g., temperature, pH, mixing speed) and output responses (e.g., yield, purity, CQA).
Benefits of DoE:
- Optimization of process parameters
- Determination of design space
- Robust process understanding
3. Multivariate Data Analysis (MVDA)
Helps in understanding the interaction among multiple variables in large datasets, often used in conjunction with PAT and DoE.
4. Control Charts & Statistical Process Control (SPC)
Used to monitor process stability and capability.
Lifecycle Approach to QbD
QbD is not limited to development alone; it is a lifecycle approach that encompasses:
- Pharmaceutical Development (ICH Q8)
- Quality Risk Management (ICH Q9)
- Pharmaceutical Quality System (ICH Q10)
- Knowledge Management (ICH Q12)
Regulatory Support for QbD
International regulatory agencies such as the FDA, EMA, and PMDA strongly encourage the adoption of QbD principles.
Key Guidelines
- ICH Q8(R2) – Pharmaceutical Development
- ICH Q9 – Quality Risk Management
- ICH Q10 – Pharmaceutical Quality System
- ICH Q11 – Development and Manufacture of Drug Substances
Advantages of QbD
- Enhanced understanding of processes and products
- Improved product quality and consistency
- Reduced batch failures
- Lower regulatory burden
- Faster approval timelines for post-approval changes
- Improved customer and regulatory confidence
Challenges in Implementing QbD
- Requires significant investment in training and infrastructure
- Resistance to change from traditional approaches
- High initial cost and resource demand
- Regulatory uncertainty during the transition phase
Conclusion
Quality by Design is a transformative approach in pharmaceutical development, aiming for a deeper understanding of processes and the proactive design of quality into products from the outset. It facilitates a lifecycle approach, promotes innovation, and ensures that quality is not just tested but is built into the product. With growing regulatory endorsement and industry-wide adoption, QbD is shaping the future of robust, efficient, and scientifically sound drug development.

